Democritus
400 BCE
Democritus' Theory
 Democritus (460 BCE - 370 BCE). Painting by Jusepe de Ribera, 1630 CE.
Democritus (460 BCE - 370 BCE). Painting by Jusepe de Ribera, 1630 CE.As with many discoveries, the atom can be traced back to philosophical origin. The early Greek philosophers Leucippus and Democritus formed the basic atomic theory that would later prove to be the foundation of modern understanding of the world. The two philosophers are considered to be the first “atomists.” “Atomism,” is defined as the theory that minute, discrete, finite, and indivisible elements are the ultimate constituents of all matter. Basically, it's synonymous with atomic theory. Some dispute that atomism was present before Leucippus and Democritus, but this claim is not backed by much historical evidence.
Not much is known about Leucippus, except that he was Democritus’ teacher. One can, however, extrapolate what Leucippus believed from Democritus’ thoughts, as Democritus is said to have taken over, organized, and expanded on his teacher’s theory.
These early atomists believed that the natural world consists of two fundamental, individual bodies: “atoms,” and the “void.” They described atoms as the limit of physical division – that is, the smallest particles that exist. The word “atom” itself was rooted in the Greek adjective “atomos,” which literally means “uncuttable.” Conversely, Leucippus and Democritus defined the void as the exact opposite of atoms – simply as nothingness.
As they are the limit of division, atoms were thought to be inherently unchangeable. According to these early philosophers, different types of atoms could be distinguished through their shape, size, order, and position. They would be in constant motion, and, depending on their shape, could temporarily bond with one another (but couldn’t permanently fuse) through the hooks and barbs on their surfaces. On a more macroscopic scale, they explained that the differences in properties among tangible materials resulted from differences between atoms and the amount of “void” between them.
Democritus applied his idea to other aspects of philosophy, like the origin of life, and the nature of truth.
Atomic theory also presented a solution to several philosophical problems of the time. Very notably, Zeno of Elea suggested that, if measurements can be divided into infinity, it would be impossible for motion to occur. Consider, for example, a runner that is trying to catch up to a tortoise in a race, where both are moving at a constant, linear speed. The runner, naturally, would be moving considerably faster than the tortoise. Yet, in the time it takes for the runner to catch up to the tortoise’s original position, the tortoise will have moved forward some distance. The runner would then attempt to move that distance, but in the time it takes them to do that, the tortoise has, once again, moved forward another distance. Theoretically, this process would repeat infinitely, and the runner would never catch up to the tortoise. Motion would be impossible unless there is a limit to division. While some philosophers (namely Parmenides) responded to the paradox by considering motion itself to be an illusion, Democritus resolved it through his atomic theory.
Democritus' Model
The key points that Leucippus and Democritus held, in regards to the atomic model, were that atoms were indivisible, unchangeable physical existences. They were believed to vary among themselves in size, shape, order. In space where atoms did not occupy, they explained, existed nothing: a “void.” The amount of void between atoms, as well as the physical properties of the atom, would, in turn, give macroscopic materials their properties.
Although, as we can tell with a couple thousand years of hindsight, his atomic theory was not completely true, his ideas paved the way for the modern understanding of the atom.
Dalton
1803
Dalton's Theory
 John Dalton (1766 - 1844) engraving by William Henry Worthington. June 25, 1823.
John Dalton (1766 - 1844) engraving by William Henry Worthington. June 25, 1823.Modern atomic theory can now be traced back to John Dalton, an English chemist and schoolteacher that provided experimental evidence to Democritus’ claims. This occurred some 2000 years after Democritus, due in part to Aristotle’s refuting of Democritus’ theory.
Attempts to trace exactly how John Dalton developed his atomic theory, however, have proven futile. Even Dalton himself had incomplete recollections on the subject. We do know that Dalton completed several experiments that provided evidence of the existence of atoms.
For instance, Dalton developed the law of multiple proportions by expanding on the work of Antoine Lavoisier and Joseph Proust. Dalton realized that 100 grams of tin could combine with either 13.5 or 27 grams of oxygen to form a tin oxide. The key here is that 27 grams is exactly double 13.5 grams. An atomic theory of matter could explain this occurrence, by showing that tin could either bind with some number of oxygen atoms (the 13.5g case), or combine with exactly twice that number of oxygen atoms (the 27g case).
Dalton had also investigated water's absorption of different gases. An atomic theory could explain why water absorbed different gases in different proportions. For example, Dalton observed that water would absorb carbon dioxide far better than it would absorb nitrogen. He inferred that this was due to differences in mass and complexity of the two gases' particles.
These key inferences, along with experimental evidence, led to his version of atomic theory, consisting of four main points:
- Everything is comprised of tiny, indivisible particles called atoms.
- Atoms of the same element are identical to each other, but are different from atoms of other elements.
- Atoms of elements can be physically mixed together, or can be chemically combined in integer ratios to form compounds.
- Chemical reactions can join, separate, or change the arrangement of an assortment of atoms. The atoms themselves retain their identity throughout this reaction.
Dalton's theory was quickly adopted by many scientists, and eventually became one of the most important theories in all of science.
Dalton's Model
To accompany his set of theories, John Dalton proposed a model of the atom. He described atoms as solid, hard spheres that were, like Democritus had said, the smallest physical unit. Unlike Democritus’ model, however, Dalton’s “billiard ball” model consisted of only spheres. Dalton also incorporated a more robust theory in regards to chemical reactions and physical mixtures of different substances.
Thomson
1897
Thomson's Theory
 JJ Thomson (1856 - 1940), photo from before 1915.
JJ Thomson (1856 - 1940), photo from before 1915.- Metal electrodes connected to a high-voltage current. Allows a beam of particles to flow across the tube.
- A positively charged electric plate.
- A negatively charged electric plate.
In 1897, English physicist Joseph John Thomson discovered the first subatomic particle – what he called “corpuscles” (now known as electrons) – through his experiments with a cathode ray tube.
We have known that sparks can travel through low-pressure gas since the time of Benjamin Franklin. The cathode ray tube effectively expands on this by allowing a current to consistently pass through a gas. To accomplish this, two metal disks are fixed at each end of a glass tube. The gas within the tube is kept at a very low pressure, and the metal disks are connected to a high-voltage electric current. After these conditions are met, a glowing beam called a “cathode ray” appears in a straight line between the two metal disks.
The question became: What exactly is the cathode ray? Scientists had been working to uncover the answer even before Thomson, but the scientific community had yet to come to a consensus. Some considered the cathode ray to consist of negatively-charged gas particles, while others believed it consisted of waves that could be deflected by magnetic fields.
First, Thomson attempted to separate the charge of the ray from the ray itself through the use of magnets. While magnets did disrupt the path of the ray, he did not detect an electric charge from where the ray used to be. This indicated that the charge and the ray were one in the same.
Although all previous attempts at deflecting the cathode ray with an electric current failed, Thomson thought of a new approach. He hypothesized that the traces of gas remaining in the tube prevented the cathode ray from being deflected by electric currents. To prove this, he was forced to improve the design of the cathode ray tube itself. Eventually, his design was able to extract nearly all of the gas from the tube, and he became the first to bend a cathode ray with an electric current.
From these two experiments, Thomson concluded that, “the [cathode rays] are charged of negative electricity carried by particles of matter.” Still, he wondered what the particles were.
- Metal electrodes connected to a high-voltage current. Allows a beam of particles to flow across the tube.
- A positively charged electric plate.
- A negatively charged electric plate.
Thomson ran several tests to determine the nature of the particle. As the particle was much too small to measure by hand, Thomson conducted experiments that he hoped could use to approximate their mass. His findings meant that the particle was either relatively massive, or was small, carrying an enormous charge. Later, through other scientists’ experiments, Thomson was able to conclude that the new particle was small, yet carried an enormous charge.
Thomson also changed the materials used in the cathode ray tube, including both the metals used at each side of the tube and the gas within the tube. From this, he concluded that the particle was present in all atoms.
Thomson concluded:
- The cathode ray is composed of negatively-charged particles.
- The particles must exist as part of the atom.
- These subatomic particles can be found in atoms of all elements.
Thomson's Model
In 1904, Thomson compiled all this new information to create the “plum-pudding” model of the atom. He inferred that, since atoms generally have a neutral net charge, the negatively-charged electrons would be accompanied by an equal positive charge. Thomson theorized that the atom was a sphere of positive charge, with negatively-charged electrons positioned evenly throughout by electrostatic forces.
Rutherford
1911
Rutherford's Theory
 Ernest Rutherford (1871 - 1937).
Ernest Rutherford (1871 - 1937).Through his famous 1911 gold-foil experiment, Ernest Rutherford, a former student of J. J. Thomson, evolved the atomic model once again.
Rutherford and his colleagues fired alpha particles, helium atoms that had lost their two electrons, at a piece of gold foil only 0.00004 cm in width. They set up a screen coated in zinc sulfide that would both “catch” the alpha particles and emit a flash of light when doing so. Because of this, after acclimating their eyes to darkness, the scientists were able to visually observe the extent in which the gold foil affected the alpha particles’ path.
Based on the prevailing atomic theory at the time, and based on the alpha particles’ considerable speed and mass, Rutherford predicted that the particles would pass through the foil, being only slightly scattered due to collisions with the gold atoms. Basically, he anticipated the particles to penetrate the foil in the same way a bullet would penetrate a bag of sand. His initial experiment confirmed this hypothesis.
Rutherford’s assistant, Hans Geiger, suggested that a research project be given to another member of their lab, Ernest Marsden. Rutherford suggested a follow-up experiment to determine if alpha particles could be deflected at large angles. Surprisingly, Marsden found that a small fraction (around 1/20000) of the alpha particles were deflected at angles larger than 90°. Famously, Rutherford recalled, “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Rutherford concluded that the positive charge and mass of an atom are concentrated in a small fraction of the total volume. He derived equations to predict the scattering that would occur for similar experiments. These were later verified by his colleagues.
Rutherford completed a new theory of the atom. He suggested that the atom is mostly empty space, with most of its mass and positive charge in the “nucleus.” This was supported by his gold-foil experiment. As it was explained, the majority of particles that passed through the gold foil without any deflection simply did not come close enough to a gold nucleus such that it could disrupt the alpha particles’ path (represented by the yellow line in the diagram). The alpha particles that were deflected at angles less than 90° came close to a gold nucleus, such that its positive charge repelled the alpha particle’s positive charge (represented by the green lines in the diagram). The particles that were reflected at angles greater than 90° directly collided with nuclei of gold atoms (represented by the blue line in the diagram).
Rutherford's Model
As perhaps one of the most drastic changes to the atomic model, Rutherford proposed an atom that was mostly empty space, with electrons orbiting around a positively-charged, dense nucleus. He estimated that the radius of the nucleus was at least 10,000 times smaller than that of the entire atom. In 1920, Rutherford proposed the name “proton” for the positively-charged particles in the nucleus of atoms.
Bohr
1913
Bohr's Theory
 Niels Bohr (1885 - 1962), a photo from 1922.
Niels Bohr (1885 - 1962), a photo from 1922.Niels Bohr, another former student of J. J. Thomson, also revolutionized the atomic model. In 1913, shortly after Rutherford and his collaborators established the existence of the nucleus, Bohr first contributed to the emerging new idea of quantum physics.
Bohr recognized a key imperfection in Rutherford’s atomic model; according to classical physics, the electrons orbiting the nucleus would lose energy until they spiraled down into the center, collapsing the atom. In other words, according to Rutherford’s model, atoms would be unstable.
Bohr applied the idea of “quanta,” developed by Max Planck in 1901, to the atomic model. In doing so, Bohr suggested that electrons existed at set levels of energy, each at a fixed distance from the nucleus. He assumed that only a limited number of orbits, with certain energies, existed. If the electron absorbed energy, it could move farther from the nucleus, to a higher energy level. If the electron radiated energy, it would fall closer to the nucleus, to a lower energy level. It would then give off energy in the form of light, equal to the difference in energy levels of the orbits.
Bohr was forced to recognize that at least some of classical physics would not be applicable at an atomic scale. He was willing to make this concession after he realized that the behavior of the hydrogen atom, which he particularly focused on in his making of the model, could not otherwise be explained.
While his model did an excellent job of explaining the spectrum of the hydrogen atom, it could not be adapted to incorporate atoms with more than one electron.
Bohr's Model
Unlike Rutherford’s model, which had electrons orbiting the nucleus indefinitely at more random distances (a theoretical impossibility), Bohr’s model incorporated electrons orbiting the nucleus at fixed energy levels. While the model explained behavior of atoms with one electron well, it was not able to extend to other atoms.
Schrödinger
1926
Schrödinger's Theory
 Erwin Schrödinger (1887 - 1961), a photo from 1933.
Erwin Schrödinger (1887 - 1961), a photo from 1933.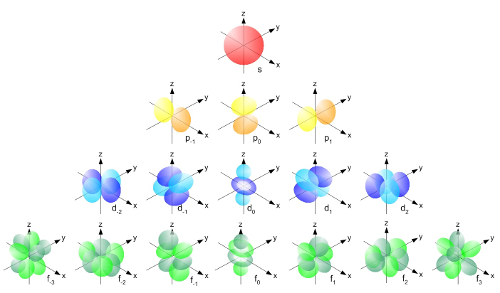 Atomic Orbitals
Atomic OrbitalsAtomic orbitals are the solutions to Schrödinger's equation.
By 1926, new theoretical calculations of the electron's orbit were inconsistent with Niels Bohr's previous description, which incorrectly depicted electrons as orbiting the nucleus like a large moving object.
Schrödinger combined equations for the behaviors of waves with the de Broglie equation to create a mathematical model describing potential electron locations within an atom. The quantum mechanical model, the most modern atomic model, comes from his solution to this equation.
Like the Bohr model, the Schrödinger model involves restricting energy levels of electrons to certain values. However, unlike the Bohr model, the Schrödinger model does not describe an exact path that electrons take, but instead describes the regions in space where electrons are likely to be under certain conditions. The quantum mechanical model also diverges from the Bohr model in that it describes electrons as waves.
The Schrödinger equation reveals the different energies an electron can have. At each energy level, the Schrödinger equation leads to a mathematical expression, called an “atomic orbital,” that represents a region in space in which there is a high probability of finding an electron.
 Atomic Orbitals
Atomic OrbitalsAtomic orbitals are the solutions to Schrödinger's equation.
The image represents all of the atomic orbitals. Different orbitals apply to different atoms. Taken as a whole, though, they represent a kind of “cloud” that describes the area where electrons are likely to be found. When people are describing “electron configurations” of an atom, they’re describing the orbitals and energy levels that apply to that particular atom’s electrons.
Schrödinger's Model
Energy levels and atomic orbitals must be adjusted to a particular atom. Taken as a whole, however, a simplified version of the quantum mechanical model is the “electron cloud” model. In this representation, the “cloud” surrounding the nucleus represents possible locations an electron can occupy. Electrons are more likely to occupy spaces where the cloud is more dense.
Typically speaking, the “cloud” represents the space where an electron is likely to be found 90% of the time. There is at least a slight chance of an electron being found beyond the cloud.